Clean Room Manufacturing – The Lowdown
October 2, 2020

In 2020, we opened a brand new fully certified Class 7 Cleanroom in response to customer demands for Cleanroom manufacturing, so we’ve been handling many enquiries about the facility recently. We’ll write soon about our own capabilities in this context and the kind of work we are doing for clients but we thought ‘d go back to basics initially and outline what a Cleanroom is, answer some common questions and discuss how cleanroom manufacturing is applied in different industries.
What is a Cleanroom?
A cleanroom (or modular cleanroom) is a controlled environment where pollutants like dust, airborne microbes, and aerosol particles are filtered out to produce a clean environment. Typically, cleanrooms are used for manufacturing products in industries where specified levels of hygiene are necessary, like the pharma, medical devices and electronic sectors.
Cleanrooms are categorised into different levels of contamination depending on the amount of particles allowed in the space per cubic meter. There’re also other important control variables such as humidity, temperature and air flow.
In summary, a Cleanroom’s core purpose is to control the introduction, generation and retention of particles inside the room.
Why use a Cleanroom?
Reason 1: Legal Responsibilities
Using a cleanroom is not just an aspiration but a must for any company that wants to improve quality control and guarantee safety in the final assembly of critical parts. For something like medical devices, this is absolutely critical. In this sector and in others like pharma, there are many different types of healthcare standards and approvals needed including USP Class V1, FDA and more. And a key component in meeting many of these legal obligations often includes the use of Cleanrooms during the manufacturing process.
Reason 2: Keep up with advanced Technology
As tech evolves, many products and their components are getting smaller and more complex (think of a smartphone circuit board, for example). As this happens, the potential negative impact and the risk of contamination in manufacturing becomes higher. So as the stakes increase and part sizes decrease, the need to prevent contamination becomes more pressing.
Reason 3: People

Yes we (human beings) are one of the main reasons why Cleanrooms are necessary. Just by simply being in a room, we are releasing particles into the air. These are mostly human skin cells that naturally shed. And the more we move the more we ‘shed’. For this reason fast movements such as running or walking fast are typically prohibited. However until we are all replaced by robots, a development which is on the horizon in manufacturing, highly trained and specialised people are still essential in areas such as injection moulding. So people need to be physically in the manufacturing facility to carry out the job.
To counteract the pollutants that workers generate, organisations like Key Plastics who offer Cleanroom manufacturing understand that it is imperative that people working in the room are following the correct protocol.
2 main considerations include:
- Covering people with protective gowns and equipment to prevent the shedding of microorganisms and
- Putting a localised protection on a given product or part to minimise contact
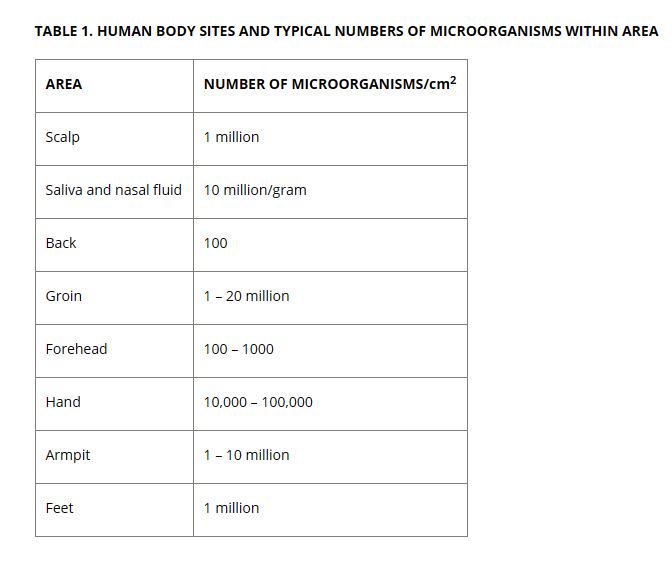
Source: IVT Network
Reason 4: Waste Reduction
When using a Cleanroom –the everyday contaminants that often happen in the manufacturing process disappear. The thoroughness of Cleanroom procedures and processes reduces the likelihood of poor production and wasted product, minimising overall waste.
Reason 5: Savings
This is really a combination of the points outlined earlier. With less waste and higher quality control (because the tailored environment allows for it) deploying Cleanroom manufacturing is fundamentally going to save on spend in the long-term.
What makes a Cleanroom Clean?
There are some important factors to consider when managing the level of airborne particles in a Cleanroom:
- Supplying the room with a large quantity of air filtered with high efficiency filters (HEPA or ULPA) to dilute and remove particles, bacteria and chemicals from within the room. The air is also used to pressurise the room. This means that any contaminated air does not flow back into the room.
- The Cleanroom itself must be built with materials that do not generate particles, contaminants or outgas airborne chemicals. These materials must also be easy to clean.
- Cleanroom workers must wear garments that minimise the dispersion of particles and micro-organisms. As mentioned above, people are one of the biggest threats to potential air contamination.
What is a Moulding Cleanroom?
A moulding Cleanroom is used specifically to produce the different types of plastics components that are required to manufacture products. It operates in line with the same principles of any other Cleanroom by eliminating the possibility of contamination and minimising particles in the air – the process is very efficient and specialised. (It is for this similar reason also that many industries such as the medical field, now use antimicrobial plastics in their products)
The medical, pharmaceutical, aerospace, military, and biotech industries often need a cleanroom to manufacture some of their parts through moulding.
These parts could be anything from medical devices to plastic bags to surgical equipment. Many of the most innovative products require precision manufacturing and robust processes.
What types of Cleanroom are there?
What are the Cleanroom classifications?
Cleanrooms are grouped & organised based on the number and size of particles permitted per volume of air. This ranges from 0.1 micron to 5 micron with the cleanrooms ranging from ISO 1 to ISO 9.
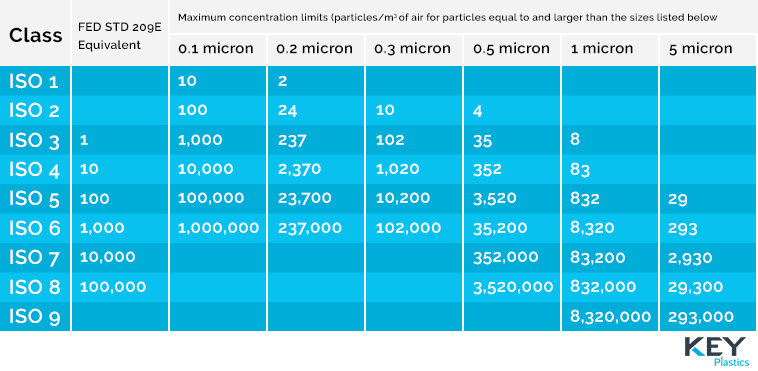
Cleanroom classifications above.
So which Cleanroom Class is right for your manufacturing requirements?
There are many factors to consider in your decision – ranging from cost, room setup (with size design) and what exactly you are manufacturing. However the starting point is to look at the legal, regulatory, manufacturing and marketing requirements for your industry.
The Pharma and Medical device industry for example have specific guidelines when it comes to selecting a Cleanroom.
Another factor to take into consideration is the Cleanroom assembly. An ideal Cleanroom setup should not just be about the injection moulding capabilities like 2k moulding but also (and importantly) – can it be used to assemble parts? This may depend on the Cleanroom provider – the size of their facilities and their capabilities. Typically many companies choose a Cleanroom according to the specifications required but also the assembly capabilities within the room. For example, if you are considering a tooling transfer, a the right cleanroom could be an important factor.
Let’s take a look at one of the most popular and effective Cleanroom types that cover’s many different industries and the one we offer here at Key Plastics – the ISO class 7 Cleanroom.
What is an ISO Class 7 Cleanroom?
An ISO Class 7 cleanroom is a type of cleanroom that is meticulously designed to prevent contamination from happening inside the facility. Typically a Class 7 Certified Cleanroom can be used for injection moulding and final assembly of critical parts for the medical device or electronics industries.
Why use ISO Class 7 Cleanroom?
The ISO Class 7 Cleanroom facilitates the needs of many different types of clients manufacturing requirements as it maintains extremely low levels of particulate matter such as dust and airborne organisms – quantified by 10,000 particles per cubic meter. The Class 7 environment is tested to ISO 14644-1 and the HEPA (High Efficiency Penetration Air) filtration rate is over 99% efficient.
Key Plastics is certified to ISO 13485- Medical Devices
An Industry Example – Medical Devices and Cleanrooms
Invasive procedures involve contact between a medical device or surgical instrument and a patient’s sterile tissue or mucous membranes. Therefore, for safety (and legal) issues – absolute precision and effectiveness is a non-negotiable when it comes to selecting a Cleanroom for medical device manufacturing.
What’s the Best Cleanroom for Medical devices?
Generally speaking medical device manufacturing is conducted in an ISO 5 – 8 Cleanroom (Class 100 – 100,000) & Medical device packaging is conducted in an ISO Class 7- 8 Cleanroom. (It is for this reason that the ISO 7 Cleanroom is a reliable and versatile choice when it comes to medical devices).
There are various polymer types that can be used in the manufacturing of medical devices. These include:
- Polyethylene (PE)
- Polypropylene (PP)
- Polystyrene (PS)
- High Impact Polystyrene (HIPS)
- Acrylonitrile Butadiene Styrene (ABS)
- Styrene Acrylonitrile (SAN)
- Polyamide – Nylon 6,66,12 etc (PA)
- Poyoxymethylene (POM- Acetal)
- Polymethylmetacrylate (PMMA- Acrylic)
- Polybutadiene Terephthalate (PBT)
- Polycarbonate (PC)
- Polyurethane (PU)
- Polyetherether Ketone (PEEK)
- Composites/Blends (Glass Filled/ Carbon fibre filled / Talc Filled etc)
- Ultim Material Blends
- Biodegradable Polymers
Final Words on Cleanrooms
Cleanrooms are a fundamental part of the manufacturing process in many sectors. Some of the worlds biggest companies such as Becton Dickinson, WEST Pharmaceuticals and Allergan all have Cleanrooms for the reasons above.
If you are interested in working with a manufacturer with ISO Class 7 Cleanrooms in Ireland who can work locally and ship internationally – please get in touch with us.



